Distillation & Evaporation
Distillation& Evaporation Plants
Explore our products
Products
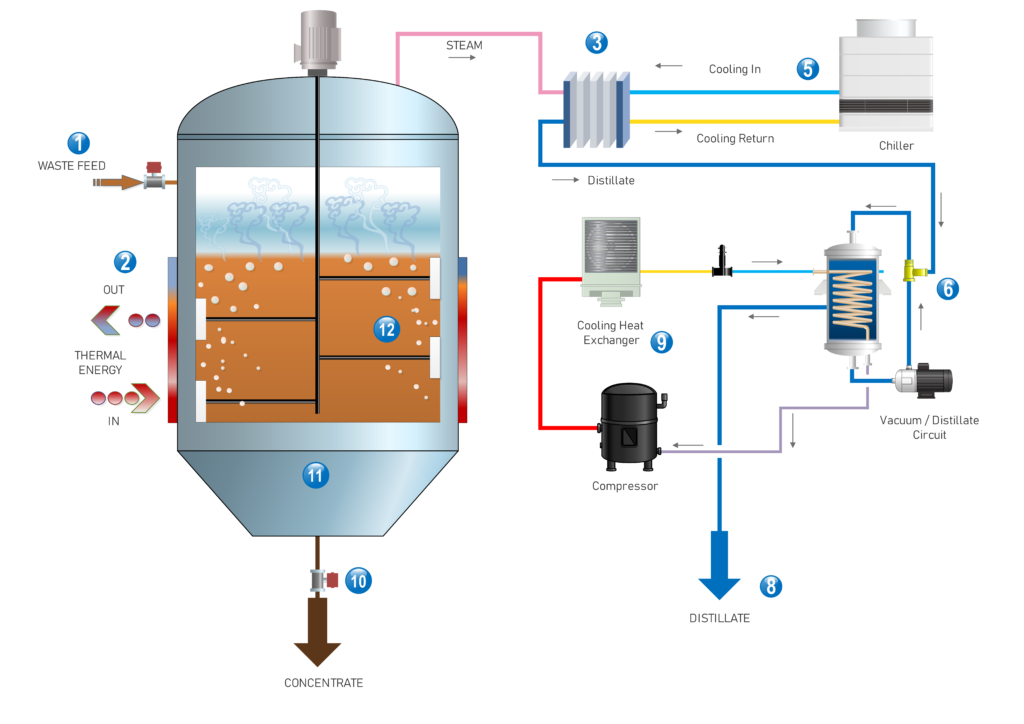
Evaporation
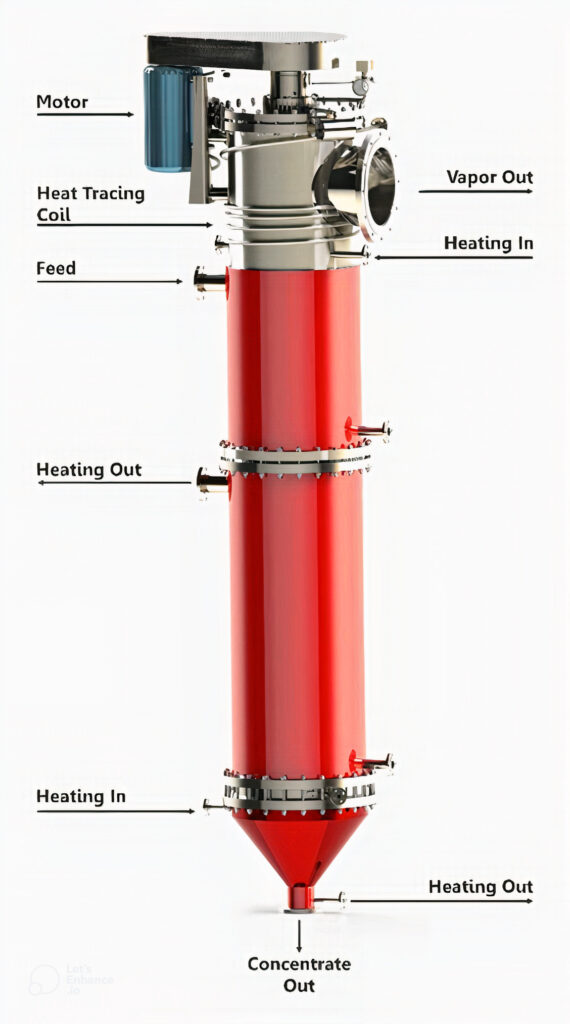
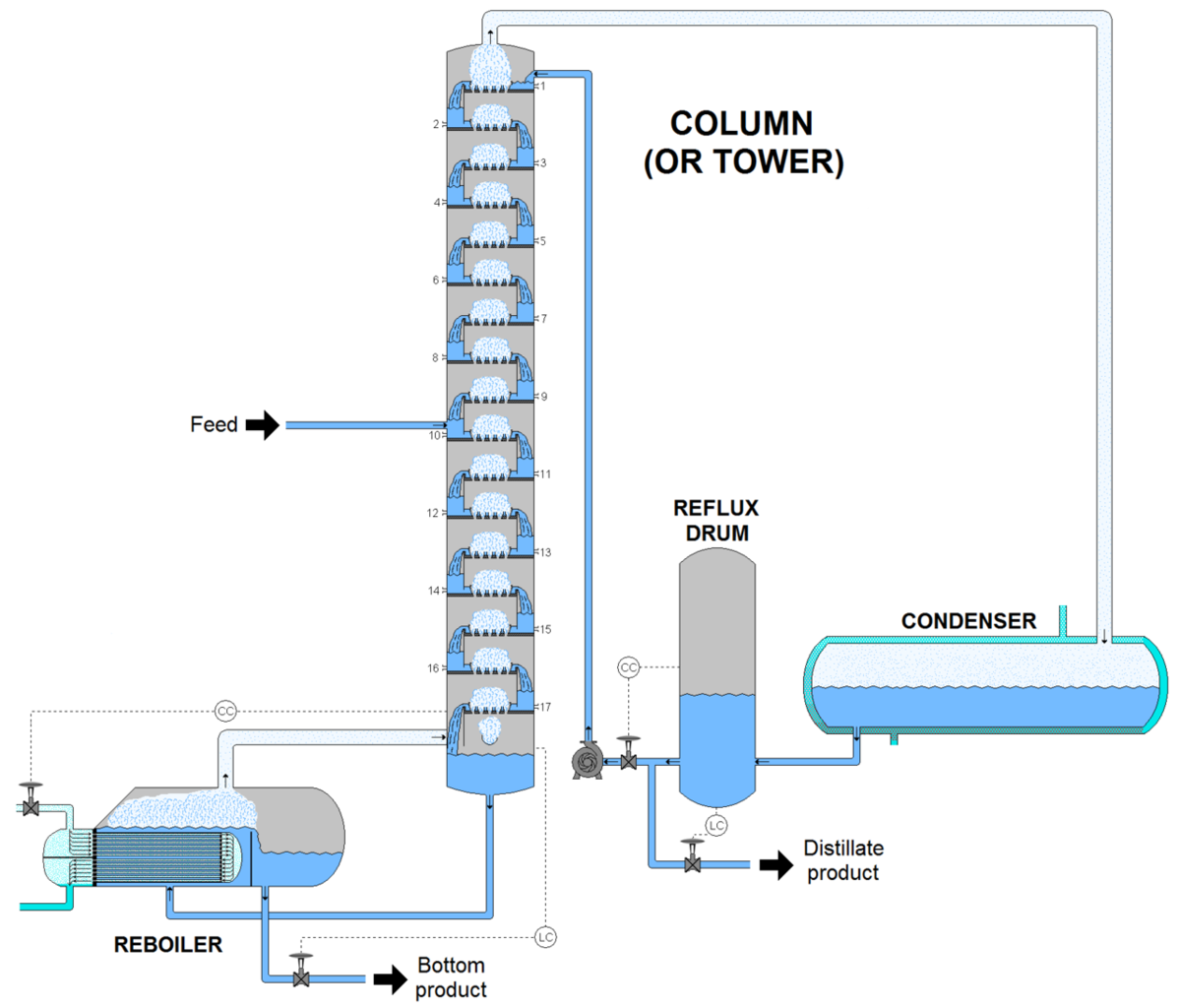
Distillation
Modern separation technique adapted based on the different boiling points of liquids. This is due to the strengths of different intermolecular forces of substances. Different liquids boil at different temperatures because the heat energy needed for bond breaking varies.
Distillation is used to separate mixtures of liquids. This involves boiling and condensing liquids.
The liquid is heated and boiled at its boiling point. The temperature remains constant until the relevant liquid vaporizes completely. The vapour is then turned into liquid phase with the aid of a condenser.
There are several distillation methods such as simple distillation, fractional distillation and steam distillation.
Simple Distillation
This is used to separate liquids with a significant boiling point gap. The components of the liquid mixture are separated when they boil at their respective boiling points and change into the vapour phase. The vapour is then condensed and collected.
Fractional Distillation
In this methods, a fractionating column is employed to separate two miscible liquids, which have close boiling points.
Steam Distillation
Steam is used to separate compounds which are immiscible with water. When such compounds are mixed with steam, they tend to vaporize at a lower temperature than their usual boiling point